
Indications for heart transplantation
Recipient criteria for heart transplantation include, severe symp- toms despite maximal medical management, the absence of reversible or surgically amenable heart disease, and where esti- mated 1-year survival is less than 50%.2
An estimate of functional capacity can be quantified by measurement of peak O2 consump-tion (VO2max). Currently, VO2max remains the single best cardio- pulmonary evaluation to predict mortality in heart failure. Patients with low VO2max (< 12 ml/min/kg) have high mortality even if treated with beta blockers. The recent International Society of Heart and Lung (ISHLT) guidelines suggest that transplantation should be considered for these patients.2 In addition heart failure prognosis scores to estimate survival, such as the Heart Failure Severity Score may be used. This calculates a survival probability on the basis of the presence of ischaemic cardiomyopathy, resting heart rate, left ventricular ejection fraction, mean blood pressure, interventricular conduction delay, VO2max and serum sodium concentration.3
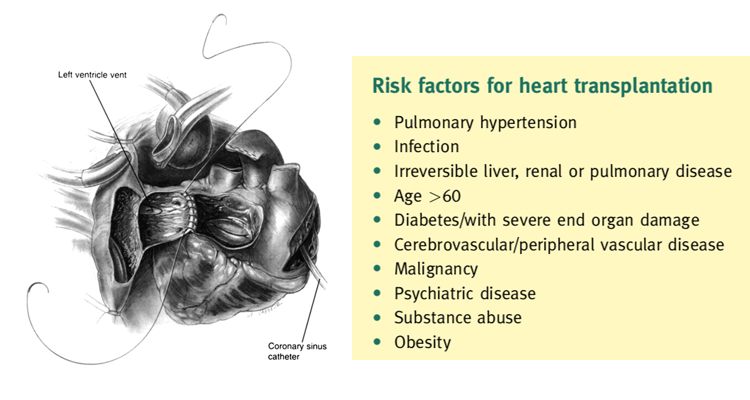
The eligibility for transplantation is considered with regard to risk factors, notably pulmonary hypertension. Right heart cath- eterization should be performed in all potential candidates for heart transplantation to quantify pulmonary vascular resistance.2
Right heart failure is a substantial cause of mortality as right ventricular failure is likely when post implant pulmonary artery pressures exceed 50 mmHg. Patients with chronic heart failure may develop pulmonary hypertension due to elevated left ventricular end diastolic pressure with elevated left atrial and pulmonary venous pressures. This is a reactive form of pulmonary hypertension and may fall when the cardiac output is increased with inotropes or unloaded with nitrate infusions.2 The transpulmonary gradient is calculated by subtracting the left atrial filling pressure from the mean pulmonary artery pressure. A fixed transpulmonary gradient in excess of 14 mmHg is associated with greatly elevated risk, and thus this cut off is used in the UK. Other important risk factors are summarized in Box 2.
Preoperative preparation
Donor-recipient matching takes place on the basis of urgency, blood group and size (80% or greater of recipient body weight). With regard to HLA status, organs are not used when the recipient has preexisting antibodies to the donor’s HLA antigens. The donor heart is assessed by measurement of filling pressures and cardiac output with a Swan Ganz catheter inserted by the organ retrieval team or by direct pressure measurements. Transoesophageal echocardiography is sometimes used to support the retrieval assessment process. Conditions precluding use of a donor heart are summarized.
If the donor heart is deemed to be satisfactory, the patient is prepared for surgery. Immunosuppression is given preoperatively, azathioprine and cyclosporine orally 24 h preoperatively as well as anaesthetic premedication.
Donor retrieval for heart
The retrieval process is a highly organized process as a part of multi disciplinary, multi-organ retrieval. The assessment takes place with 96 the assistance of a Swan Ganz catheter and occasionally with transoesophageal echocardiography to assess function and the presence of valvular disease or other anomalies. After median sternotomy, the pericardium is opened and elevated and the heart is inspected and palpated for size, contractility and anomalies. The coronary arteries are assessed for damage or palpable coronary artery disease. The inferior vena cava (IVC), superior vena cava (SVC) and ascending aorta are dissected. Heparin is administered.
The retrieval commences by cross clamping the aorta as high as possible. Cardioplegia solution (15 ml/kg) is administered via a cannula in the ascending aorta until asystole occurs. The heart is cooled with topical ice-cold saline. The IVC is divided and an incision is made in the left atrial appendage especially if the lung is concurrently retrieved to prevent distension of the LV. After giving cardioplegia, the aorta is divided as high as possible. The pulmonary artery is divided at the level of its bifurcation. The SVC is divided at the level of the azygos vein. If the heart and lungs are to be retrieved, the left atrium is incised at the junction of the left superior pulmonary vein, and extended inferiorly. Care is taken to avoid injury to the coronary sinus. The incision continues inferiorly and then to the junction of the right inferior pulmonary veins and left atrium. By this means the heart is excised with a cuff of left atrium and adequate cuff of left atrium is left continuous with the pulmonary veins to facilitate lung retrieval and subsequent implantation. If the heart alone is to be retrieved, then the pulmonary veins are divided and the intact left atrium is left in continuity with the retrieved heart. Meticulous preservation by administration of adequate cardioplegia and topical cooling is required. The retrieved heart is stored in ice-cold saline, triple bagged and transported in ice to ensure adequate preservation. It is important to expeditiously transport the heart to the implant centre to ensure a tolerable ischaemic time. There is an inverse relationship between ischaemic time and post-transplant survival.4
Heart transplantation: operative steps
Through a mid-line sternotomy, the diseased heart is exposed. Following full systemic heparinization, cannulation for cardiopulmonary bypass is accomplished with a straight aortic cannula, high in the ascending aorta. Venous cannulation is via a right angle or straight cannula directly into the superior vena cava or through the posterior right atrium into the superior vena cava. Inferior vena cava cannulation is accomplished through the posterior right atrium. Certain situations, such as recipient instability or difficult medias- tinal dissection may need femoral cannulation for cardiopulmonary bypass.
When the donor heart is close to the operating room the ascending aorta is cross-clamped and the diseased heart is excised. An incision is made in the right atrial appendage. This is extended inferiorly, anterior to the inferior vena cava cannula and towards the aorta root. The left atrium is entered through the superior limb of the fossa ovalis. The aorta and pulmonary arteries are transected just above their ventriculo-arterial valves. The interatrial septum is divided down to the coronary sinus, which demarcates the atrio- ventricular groove. Resection of the heart results in cuffs of left atrium, SVC, IVC, aorta, and pulmonary artery.
Prior to implantation, the donor heart is inspected for a patent foramen ovale, which is closed by direct suture, and any other anomalies. Further myocardial protection in the form of 1000 ml of cold blood cardioplegia may be given into the donor heart via its clamped aortic root.
The implant procedure begins with anastomosis of donor and recipient left atria5 (Figure 1). Using a 4/0 polypropylene monofilament suture, the anastomosis starts at the level of the left atrial appendage. Care is taken to align the interatrial septum. A transmitral vent is placed prior to completion of the left atrial anastomosis to vent the left ventricle and stop it from distending. Cardiac cooling may be augmented by running cold Ringer’s solution through this vent during implantation. The next anastomosis is to the donor and recipient aorta. The donor aorta is left long so that there this is little tension on the anastomosis, and so it can be manipulated to allow inspection of the suture lines on completion of the transplant. This is done with continuous 4/0 polypropylene monofilament suture. At this point the heart can be deaired via a needle vent placed in the ascending aorta, and reperfusion begun with warm, leukocyte filtered blood at a carefully controlled pressure. Prior to release of the cross clamp, 500 mg of methylprednisolone is given intravenously. The next anastomosis is the PA, this is done with continuous 5/0 polypropylene. The pulmonary artery should be trimmed short. If the pulmonary artery is left too long, distortion can occur with re-establishment of normal right ventricular contraction. The final anastomoses are between the respective inferior and superior vena cavae,6 with continuous 5/0 polypropylene, tied down initially to prevent purse stringing of these low pressure structures.
When the patient has been rewarmed there follows at least 30 min of reperfusion. An isoprenaline infusion is commenced, pacing wires are placed on the surface of the heart and the patient is weaned from cardiopulmonary bypass. Protamine is administered to reverse the effects of heparin and haemostasis is performed.
The patient is managed in an intensive care environment in an isolated room. The patient is rewarmed and ventilatory requirements are progressively weaned. There is careful monitoring of cardiac function and volume status. This may be done with a Swan Ganz catheter or a left atrial line placed at the time of the transplant. Transoesophageal echocardiography is also used to monitor graft function and to rule out a pericardial collection or tamponade, if suspected. Immunosuppression consists of methylpredisolone 125 mg given 8 hourly for three doses and then the institution of cyclosporine at 1-2 mg/kg if there is no or minimal renal dysfunction. If there is renal dysfunction preoperatively, then a cyclosporine sparing regimen is possible using antithymocyte globulin with later institution of the calcineurin inhibitor. Eventually, the patient is established on a stable regimen of oral corticosteroid, calcineurin inhibitor, typically ciclosporin or tacrolimus, and an antimetabolite, usually azathioprine or mycophenolate. The International Society of Heart and Lung Transplantation (ISHLT) have issued guidelines which cover all subsequent management.7
Results
The registry database is managed by the International Society of Heart and Lung Transplantation. Since 1967, in excess of 88,000 total heart transplants through to the end of March 2010.4 In this cumulative registry, 1-year survival is 81%. Thereafter the annual mortality is 4% per year. The half-life for survival is 10 years. Hazards are highest in the first year of transplant, after this the conditional half-life is 13 years. Analysis has shown that more recent cohorts have a better survival. The causes of death within the first 6 months are mainly due to graft dysfunction and infection. Late attrition is due to chronic rejection, in particular chronic allograft vasculopathy and malignancies. Improved outcome by era of transplantation has been shown and is due to a number of factors including the emergence of calcineurin inhibitors, lower chronic steroid use, earlier diagnosis of rejection and better patient selection.