The most important trial of CABG versus PCI is the SYNTAX Trial.
SYNTAX was a trial in patients with multi-vessel and/or left main stem disease.
SYNTAX also had a database called a nested parallel registry that looked at outcomes in 1,078 patients who were deemed ineligible for randomisation for the SYNTAX study. Over 80% of the patients in this registry had coronary artery disease of such severity that it was considered they would not be appropriate candidates for stenting and were therefore referred directly for CABG. Only 16% of the registry patients actually underwent PCI having been deemed prohibitively high risk for surgery.
Overall, at one-year, the main result of SYNTAX was that repeat revascularization was much higher for those patients undergoing stent implantation compared to surgery(5.9% vs. 13.5%; P<0.001).
Overall, at five-years, stent implantation/ PCI failed to reach the pre-specified criteria for non-inferiority. In 1095 patients with three-vessel CAD, CABG reduced the risk of death (9.2% vs. 14.6%; P=0.006), MI (3.3% vs. 10.6%; P<0.001) and need for repeat revascularization (12.6% vs. 25.4%; P<0.001) without an increase in the risk of stroke (3.4% vs. 3%; P=0.66).
When analyzed by severity of CAD, as judged by SYNTAX scores, patients with intermediate (between 23-32) and higher (>32) scores had an absolute survival advantage with CABG as well as highly significant reductions in the incidence of MI and need for repeat revascularization.
Only in those with scores <22 was there a similar mortality between CABG and PCI, although CABG still resulted in significantly fewer MI and repeat revascularization.
This is an important distinction as 79% of all patients with three-vessel CAD in SYNTAX (1,095 in the RCT and 570 in the registry) had SYNTAX scores >22.
However, when the SYNTAX results are analyzed according to patients with left main disease a different pattern of emerges. In contrast to the situation for three-vessel CAD the respective 5-year rates of death (14.6% vs. 12.8%; P=0.53) and MI (4.8% vs. 8.2%; P=0.10) were similar whereas CABG had a lower risk of repeat revascularization in patients with SYNTAX scores less than 32.
In patients with SYNTAX scores >32 CABG resulted in lower mortality (14.1% vs. 20.9%; P=0.11) and the need for repeat revascularization (11.6% vs. 34.1%; P<0.001).
The optimal revascularization strategy in patients with diabetes has been settled by the Freedom trial. This trial randomized 1900 patients with diabetes and multivessel CAD already receiving aggressive medical therapy, to CABG or DES. The 5-year primary composite outcome occurred in 26.6% of the PCI group and 18.7% of the CABG group (P=0.005). Crucially, the benefit of CABG was driven by highly significant absolute reductions in both death (5.4%; P=0.049) and myocardial infarction (7.9%; P<0.001).
Patients with symptomatic severe valvular AS have a POOR PROGNOSIS WITHOUT VALVE REPLACEMENT.
The average survival rate was 3 years after the onset of angina pectoris in patients with severe Aortic Stenosis (AS). The average survival rate after the onset of syncope in patients with severe AS ISs 3 years. The average survival rate after the onset of heart failure in patients with severe AS was 1.5 to 2 years. Surgical Aortic Valve Replacement (AVR) is the procedure of choice for symptomatic patients with severe AS (an aortic valve area less than 1.0 cm2) with a Class I indication.
In a prospective study, at 19-month follow-up (range 2 to 36 months), 90% of 30 patients with heart failure associated with unoperated severe AS and a normal left ventricular ejection fraction were dead [3]. At 13-month follow-up (range 2 to 24 months), 100% of 18 patients with heart failure associated with unoperated severe AS and an abnormal left ventricular ejection fraction were dead. In a prospective study, at 20-month follow-up of 40 elderly patients with severe AS, heart failure, syncope, or angina pectoris was present in 36 of 37 patients (97%) who developed new coronary events and in none of 3 patients (0%) without new coronary events [4].
Other Class I indications for AVR in patients with severe AS include patients undergoing coronary artery bypass graft surgery, patients undergoing surgery on the aorta or other heart valves, and patients with a left ventricular ejection fraction less than 50%. Patients with moderate AS undergoing coronary artery bypass graft surgery or surgery on the aorta or other heart valves have a Class IIa indication for AVR.
The natural history of chronic aortic regurgitation is well recognized. The asymptomatic patient who has moderate to severe aortic regurgitation may not have symptoms for many years. In seven studies,1–7 490 asymptomatic patients with moderate to severe aortic regurgitation were followed for a mean of 6.4 years. Based on these studies, the following can be concluded about asymptomatic chronic aortic regurgitation with normal left ventricular systolic function (i.e., an ejection fraction of 50 percent or greater)8:
In patients with asymptomatic chronic aortic regurgitation and left ventricular dysfunction, the rate of progression to symptoms is higher than 25 percent per year. In symptomatic patients, the mortality rate is greater than 10 percent per year.8
Although there is only a small chance of sudden death or left ventricular dysfunction in patients with asymptomatic chronic aortic regurgitation, these adverse events are possible. Therefore, it is important to follow asymptomatic patients for the development of symptoms and to examine them regularly to detect progression of severity. In addition, periodic quantitative evaluation of left ventricular function is necessary in patients with moderate to severe aortic regurgitation. When the history of exercise tolerance or symptoms is equivocal, exercise stress testing can be helpful.
The presence of aortic regurgitation, even when it is moderately severe, is not necessarily an indication for surgery. When surgery is needed, aortic valve replacement or repair, is usually required. Prosthetic valves, whether mechanical or biologic, have problems with durability, paraprosthetic leaks, thromboembolism and increased susceptibility to infective endocarditis. The decision to replace the valve requires that these potential problems be considered to represent less danger to the patient’s well-being than continuing medical management.
When should surgery be done for Aortic Regurgitation?
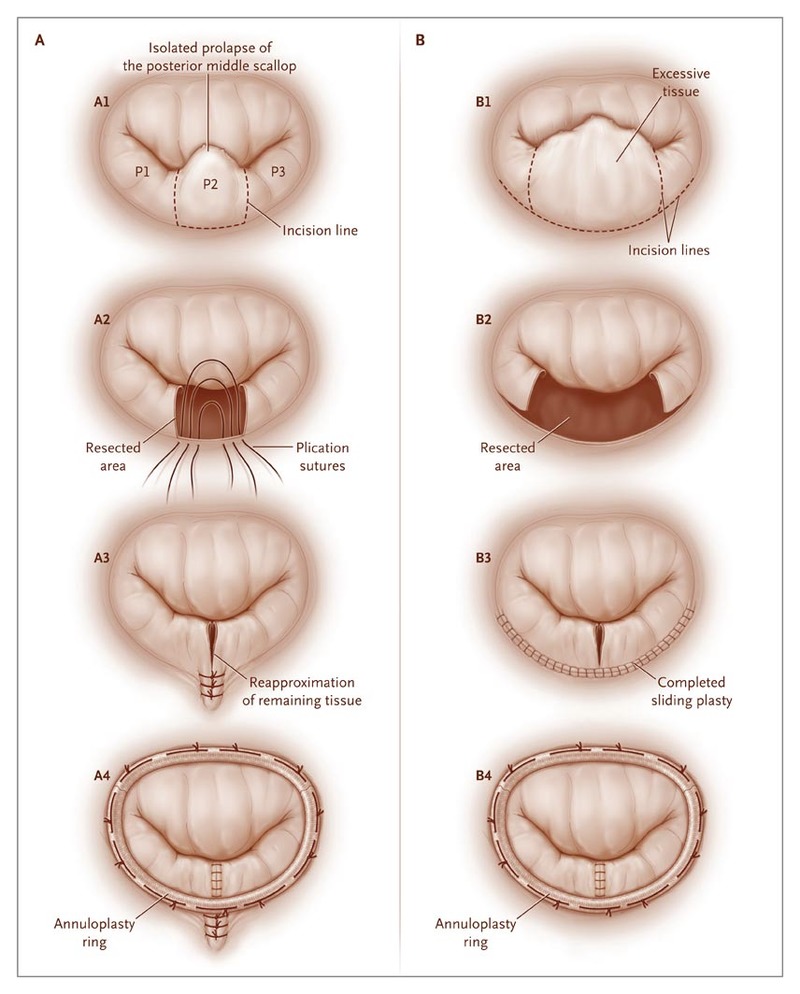
All patients who have any symptoms and moderate or severe mitral regurgitation should be referred for surgical treatment. It is preferable to operate on patients early in their symptomatic course, as long term survival following mitral valve repair is compromised in patients with New York Heart Association Class III or IV symptoms (see Figure 1)2. Factors which determine timing of surgery for degenerative mitral regurgitation in the current European Society of Cardiology American College of Cardiology and American Heart Association and guidelines include the presence of symptoms, left ventricular (LV) ejection fraction (EF), LV end-systolic dimension (LV ESD), atrial fibrillation, and pulmonary hypertension1.
Dr Babar Bashir CHAUDHRI works in concert with expert cardiologists to evaluation patients with severe mitral regurgitation. Patients with symptoms, who have been found to have lesser degrees of regurgitation by prior investigations elsewhere will often be found to have increased mitral regurgitation, or inadequate increase in ejection fraction, on stress echocardiography and should also be referred for surgical consideration.
Asymptomatic patients with left ventricular dilatation (LV end systolic diameter more than 45 mm), decreased Ejection Fraction (<60%), atrial fibrillation or pulmonary hypertension (PA systolic pressure > 50 mm Hg at rest or > 60 mm Hg with exercise) should also be considered for elective mitral valve surgery.
Patients with asymptomatic moderate to severe mitral regurgitation should be carefully followed up. Attention is focused to repeated measurement of ejection fraction over the course of time. A drop to <60% confers poorer long term survival even with successful mitral valve repair. Patients with severe quantitative mitral regurgitation (effective regurgitant orifice of at least 40 mm2) have significant morbidity including increased mortality during follow up and should be considered for surgery.
Dr Babar Bashir CHAUDHRI will discuss all treatment options with you and will recommend surgery to all symptomatic patients and also generally to all asymptomatic patients with severe mitral regurgitation.
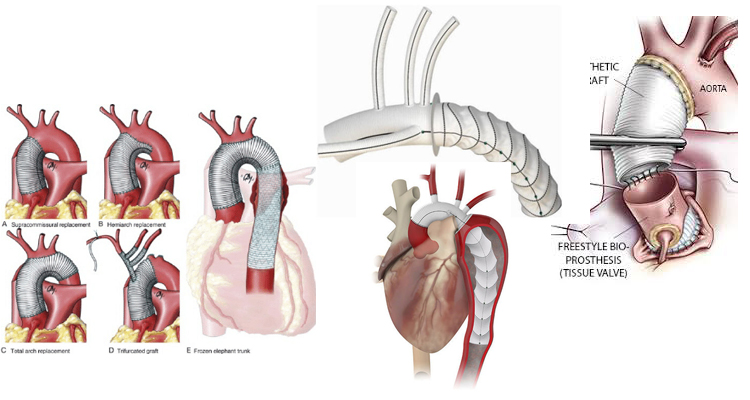
Surgical Repair of Aortic Root/ Ascending Aortic / Arch and Descending Aortic aneurysms.
Surgery for Acute Aortic Dissection
Dr Babar Bashir CHAUDHRI is an expert in the repair of aneurysms of the aorta and surgery for of acute aortic dissections.
Acute Type A aortic dissection is a catastrophic emergency, which if untreated will kill over 50% of sufferers within 48hrs. Emergency aortic surgery may include replacing the aortic valve and replacing the aortic root, ascending aorta and the arch, and the head and neck vessels. In this situation, surgery is mandated to save life.
He also has extensive experience of surgical treatment and graft replacement for aneurysms of the thoracic aorta and access to the latest devices to facilitate this (eg ThoraflexTM Hybrid: Terumo Aortic).
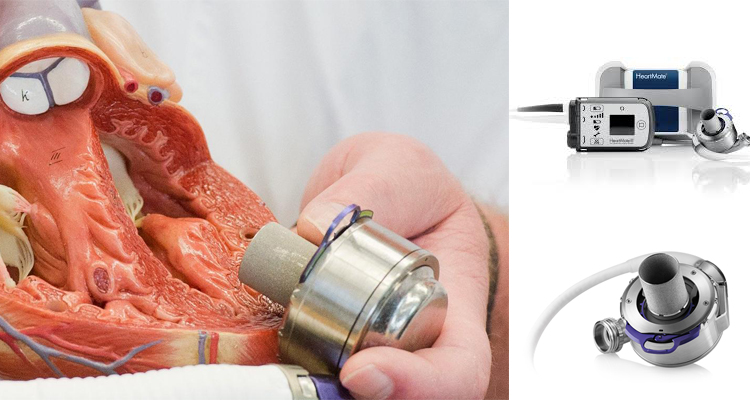
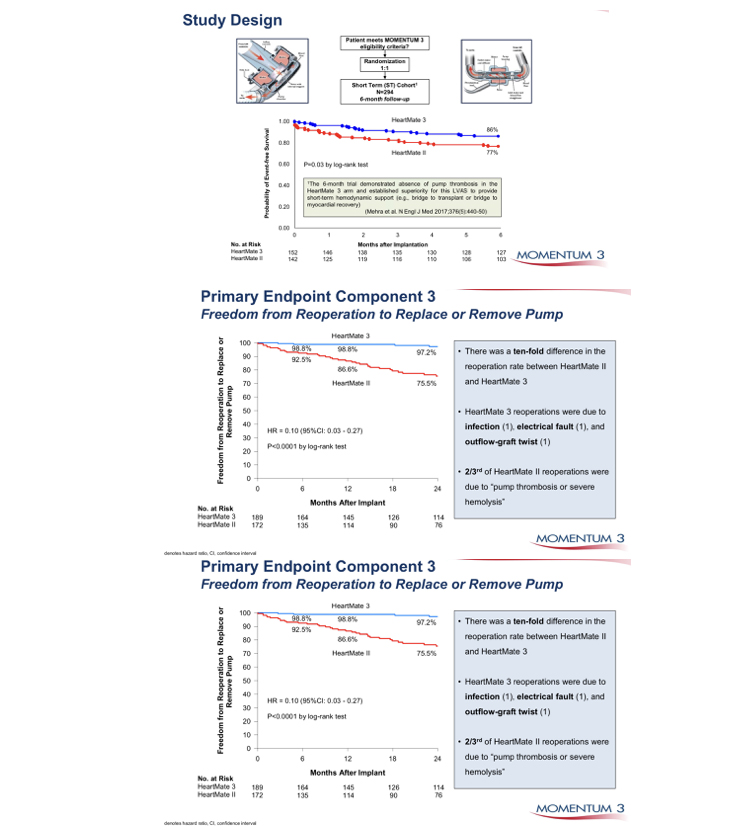
In patients with severe heart failure, cardiac transplantation has been shown to provide considerable benefit. Since 1967, in excess of 88,000 total heart transplants have been performed and 1-year survival is 81%, the annual mortality is 4% per year thereafter. The supply of donor hearts is incredibly limited and much research has focused on mechanical means of improving myocardial function, and several such left ventricular assist devices (LVADs) have been developed through the National Institutes of Health artificial-heart program. Several devices have been previously approved by the Food and Drug Administration as bridging therapy to transplantation, though none have been studied as long-term alternatives to transplantation. The Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) (1) trial explored whether a specific type of LVAD (a previous generation pulsatile device), when used in the long-term, would reduce mortality (fig 1). The survival following severe heart failure was extremely poor in the optimally medically treated group in this trial (defined as End-stage heart failure was defined as New York Heart Association (NYHA) class IV symptoms for at least 90 days, left ventricular ejection fraction (LVEF) <25%, peak oxygen consumption <12 mL/kg/min or continued need for intravenous inotropes for symptomatic hypotension).
In the optimally medically treated control arm of the Randomised Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) trial (1) which evaluated a externalised pulsation ventricular assist device, survival at one year was 28% and 6% at two years, underlying the poor prognosis of this clinical entity (fig 1).
Driving the need for mechanical circulatory support (MCS) is the relative paucity of donors and the unmet need for orthotopic heart transplantation in the general population.
There has also been an increase in the number of patients who require mechanical circulatory support (MCS) as a bridge to transplantation (2). This has been driven, particularly in the UK by limitation of the number of hearts for donation, and also to buy time on the transplant waiting list. This is due to an increase in the numbers of non-heart beating donors (DCDs), whereby retrieval takes place in a circulation arrested donor, and the increased survival of head injury patients and those with intracranial bleeds who are treated by a decompressive craniotomy, reducing the pool of donors who have raised intracranial pressure and who have coned, resulting in brain stem death. The net result is a retrieval rate for heart transplantation of around 19%. The risk of having preformed antibodies directed against the donor heart (sensitised patients) is increasingly likely and is particularly challenging as it may increase the risk of rejection and allograft vasculopathy.
There has also been an increase in the number of patients requiring MCS as a bridge to transplantation (3). This allows many severely ill adult and paediatric patients to survive until a suitable donor heart is available. Patients with MCS are at increased risk for rejection, infection, stroke, and bleeding. The need for transfusions also increase the risk of pre-sensitization (3-5).
Survival at 1 and 5 years is decreased in patients requiring MCS prior to transplantation, but still higher than 80% and 70%, respectively (ISHLT database) (2).
Advances in Donor Allocation and Selection
Recipient criteria for heart transplantation include, severe symptoms despite maximal medical management, the absence of reversible or surgically amenable heart disease, and where estimated 1-year survival is less than 50% (6). An estimate of functional capacity in ambulatory patients can be best quantified by measurement of peak O2 consumption (VO2max). Patients with low VO2max (< 12 ml/min/kg) have high mortality even if treated with beta blockers and transplantation should be considered for these patients. In addition heart failure prognosis scores to estimate survival, such as the Heart Failure Severity Score may be used. This calculates a survival probability on the basis of the presence of ischaemic cardiomyopathy, resting heart rate, left ventricular ejection fraction, mean blood pressure, interventricular conduction delay, VO2max and serum sodium concentration (7).
Transplantation eligibility is always considered with regard to risk factors, especially, pulmonary hypertension. Right heart catheterisation must be performed in all potential candidates for heart transplantation in order to quantify pulmonary vascular resistance (7). Right heart failure is a substantial cause of mortality. Right ventricular failure is likely when post implant pulmonary artery pressures exceed 50 mmHg. Patients with chronic heart failure may develop pulmonary hypertension due to elevated left ventricular end diastolic pressure with elevated left atrial and pulmonary venous pressures. This is a reactive form of pulmonary hypertension and may fall when the cardiac output is increased with inotropes or unloaded with nitrate infusions (7). The transpulmonary gradient is calculated by subtracting the left atrial filling pressure from the mean pulmonary artery pressure. A fixed transpulmonary gradient in excess of 14 mmHg is associated with greatly elevated risk, and thus this cut off is used in the UK (8). In such patients a destination therapy strategy may be used with continuous flow LVADS.
Mechanical Circulatory Assist Devices
In recent years, the use of MCS device in treating patients with end-stage heart disease has increased significantly, as bridge to transplantation and as destination therapy for transplant ineligible candidates. This increase is based on the accumulated experience with new second-generation continuous-flow devices which show significant improvements in survival, functional capacity and quality of life (9,10).On the basis of the Heart Mate II Registry experience (1300 patients), guidelines for the clinical management of patients treated with continuous-flow devices have been published (11). Risk scoring systems, such as the Seattle Heart Failure Model (12) and the Cumulative Risk Score for 90-Day in-Hospital Mortality (13) and the Destination Therapy Risk Score have been investigated to stratify patients who might benefit from LVAD support (14).
Right ventricle failure is a leading cause of morbidity and death after LVAD implant (incidence of about 35%), and can be very difficult to predict (15,16). Various means to assess right ventricle function both pre- and postoperatively have been assessed (10). Right ventricular failure risk scores have been created that stratify the risk of right ventricular failure (RVFRS) and death after LVAD implantation. One such RVFRS found independent predictors of right ventricular failure to include vasopressor requirement, aspartate aminotransferase >80 IU/L, bilirubin >2.0mg/dL and creatinine >2.3mg/dL (15). Another study developed a score to predict RVAD need after LVAD placement, which included factors of cardiac index, right ventricular stroke work index, severe preoperative right ventricular dysfunction, creatinine, previous cardiac surgery and systolic blood pressure (16) More recently the presence of severe TR and a tricuspid annulus of >43mm and right ventricular sphericity have been proposed as predictive of occult RV failure and need for biventricular support. The Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) registry, which follows all long-term MCS systems in the United States, has defined patient profiles that can help identify risks associated with the timing of implant (Table 1) (17). In the future, the INTERMACS patient profile would be a useful tool to improve management and outcomes of patients who need VAD implant, and unify criteria for future clinical trials and devices. As more LVAD patients are listed for heart transplant, a competition has occurred for organs between stable LVAD supported registrants and less stable registrants listed UNOS status 1A or 1B (the highest categories and most at risk if not urgently transplanted). A recent study found that stable LVAD patients had significantly less 30-day risk of events compared to other status 1A patients concluding that allowance of 30 days of elective status 1A time should not be allocated to stable registrants with implanted LVADs (18). As VAD technology improves, further revisions to the allocation system will need to be recommended.
Table 2 INTERMACS Profile and Description and Timescale to MCS
INTERMACS = Interagency Registry for Mechanically Assisted
Circulatory Support; MCS = mechanical circulatory support;
NYHA = New York Heart Association.
Slaughter MS, et al. J Heart Lung Transplant 2010;29:S1–S39
Temporary MCS are available that can be implanted quickly and simply to normalise cardiac output in patients with severe acutely decompensated heart failure. The CentriMag (19), TandemHeart (20), Impella (21) and Circulite (22). Clinical trials suggest that treatment of temporary VADs does not necessarily correlate with better survival, but merely comprise a component of treatment leading to recovery, upgrade to fully implantable systems as a bridge to transplant or destination therapy, or transplantation (23,24).
Device miniaturisation, without externalized drive-lines connecting the device to a console and longer endurance will be the future trend of mechanical design for long term support. Blood pumps with magnetically levitated rotors has shown satisfactory 1-year survival (25). The smaller size and weight of the continuous-flow devices has allowed an extension of the new VADs into smaller patients. Fully wireless resonant coupling power sources are currently undergoing evaluation, which if successful will greatly reduce the incidence of drive line infections, which is the weakest point of the technology of current fully implantable systems. There is some evidence that fully implantable systems will be available in the near future to greatly improve the quality of life and to reduce the frequency of severe infections with continuous flow LVADS.
Many recent studies have focused on the reversed molecular and cellular alterations, such as improved β-adrenergic responses and decreased calcium-regulating gene expression, in patients using LVAD as a bridge to recovery therapy (26). Functional recovery has been observed in a subset of heart failure patients (26, 27). Recently, a clinical trial using clenbuterol (β-2 agonist and anabolic agent) and LVAD in refractory non-ischemic heart failure patients, reported recovery of heart function in 60% of patients (n = 20) with non-ischemic cardiomyopathy that allows the pump to be explanted (Harefield Recovery Protocol Study for Patients With Refractory Chronic Heart Failure, HARPS) (28). LVAD therapy is associated with decreased collagen turnover and crosslinking and increased tissue angiotensin II. LVAD combined with angiotensin-converting enzyme inhibition results in decreased tissue angiotensin II and collagen cross-linking, normalizes left ventricular end-diastolic pressure volume relationships and is associated with modestly higher rates of bridge to recovery (29). Other adjunctive treatments including other medications, cell or gene therapy with over expression of SERCA2a might in conjunction with VAD support provide a meaningful alternative therapy in patients with severe heart disease (30).
Conclusions
Heart transplantation is associated with excellent long term outcomes and is the gold standard solution for intractable end stage heart failure in eligible patients. What limits its impact, overall, is the limited availability of donor organs. The development of ventricular assist devices has mitigated against this, to some extent. Subsequent device iterations with further miniaturisation and continuous flow have resulted in effective bridge to transplant solutions. The presence of an externalized drive line exposes the VAD recipient to infections, however, which may precipitate urgent listing for heart transplant in the bridge to transplant candidate and may limit the life span of the destination therapy candidate. Fully implantable driveline free systems will definitely enhance the utility of these systems in these settings. As our knowledge of molecular medicine increases, manipulation of key proteins implicated in the pathophysiology of heart failure such as SERCA2a may allow some recovery of the myocardium in patients with heart failure to the extent that transplantation may be deferred or the LVAD explanted (31-35).
Indications for heart transplantation
Recipient criteria for heart transplantation include, severe symp- toms despite maximal medical management, the absence of reversible or surgically amenable heart disease, and where esti- mated 1-year survival is less than 50%.2
An estimate of functional capacity can be quantified by measurement of peak O2 consump-tion (VO2max). Currently, VO2max remains the single best cardio- pulmonary evaluation to predict mortality in heart failure. Patients with low VO2max (< 12 ml/min/kg) have high mortality even if treated with beta blockers. The recent International Society of Heart and Lung (ISHLT) guidelines suggest that transplantation should be considered for these patients.2 In addition heart failure prognosis scores to estimate survival, such as the Heart Failure Severity Score may be used. This calculates a survival probability on the basis of the presence of ischaemic cardiomyopathy, resting heart rate, left ventricular ejection fraction, mean blood pressure, interventricular conduction delay, VO2max and serum sodium concentration.3
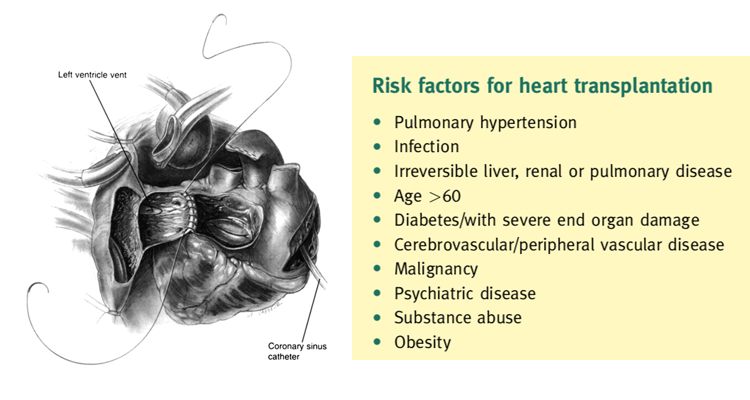
The eligibility for transplantation is considered with regard to risk factors, notably pulmonary hypertension. Right heart cath- eterization should be performed in all potential candidates for heart transplantation to quantify pulmonary vascular resistance.2
Right heart failure is a substantial cause of mortality as right ventricular failure is likely when post implant pulmonary artery pressures exceed 50 mmHg. Patients with chronic heart failure may develop pulmonary hypertension due to elevated left ventricular end diastolic pressure with elevated left atrial and pulmonary venous pressures. This is a reactive form of pulmonary hypertension and may fall when the cardiac output is increased with inotropes or unloaded with nitrate infusions.2 The transpulmonary gradient is calculated by subtracting the left atrial filling pressure from the mean pulmonary artery pressure. A fixed transpulmonary gradient in excess of 14 mmHg is associated with greatly elevated risk, and thus this cut off is used in the UK. Other important risk factors are summarized in Box 2.
Preoperative preparation
Donor-recipient matching takes place on the basis of urgency, blood group and size (80% or greater of recipient body weight). With regard to HLA status, organs are not used when the recipient has preexisting antibodies to the donor’s HLA antigens. The donor heart is assessed by measurement of filling pressures and cardiac output with a Swan Ganz catheter inserted by the organ retrieval team or by direct pressure measurements. Transoesophageal echocardiography is sometimes used to support the retrieval assessment process. Conditions precluding use of a donor heart are summarized.
If the donor heart is deemed to be satisfactory, the patient is prepared for surgery. Immunosuppression is given preoperatively, azathioprine and cyclosporine orally 24 h preoperatively as well as anaesthetic premedication.
Donor retrieval for heart
The retrieval process is a highly organized process as a part of multi disciplinary, multi-organ retrieval. The assessment takes place with 96 the assistance of a Swan Ganz catheter and occasionally with transoesophageal echocardiography to assess function and the presence of valvular disease or other anomalies. After median sternotomy, the pericardium is opened and elevated and the heart is inspected and palpated for size, contractility and anomalies. The coronary arteries are assessed for damage or palpable coronary artery disease. The inferior vena cava (IVC), superior vena cava (SVC) and ascending aorta are dissected. Heparin is administered.
The retrieval commences by cross clamping the aorta as high as possible. Cardioplegia solution (15 ml/kg) is administered via a cannula in the ascending aorta until asystole occurs. The heart is cooled with topical ice-cold saline. The IVC is divided and an incision is made in the left atrial appendage especially if the lung is concurrently retrieved to prevent distension of the LV. After giving cardioplegia, the aorta is divided as high as possible. The pulmonary artery is divided at the level of its bifurcation. The SVC is divided at the level of the azygos vein. If the heart and lungs are to be retrieved, the left atrium is incised at the junction of the left superior pulmonary vein, and extended inferiorly. Care is taken to avoid injury to the coronary sinus. The incision continues inferiorly and then to the junction of the right inferior pulmonary veins and left atrium. By this means the heart is excised with a cuff of left atrium and adequate cuff of left atrium is left continuous with the pulmonary veins to facilitate lung retrieval and subsequent implantation. If the heart alone is to be retrieved, then the pulmonary veins are divided and the intact left atrium is left in continuity with the retrieved heart. Meticulous preservation by administration of adequate cardioplegia and topical cooling is required. The retrieved heart is stored in ice-cold saline, triple bagged and transported in ice to ensure adequate preservation. It is important to expeditiously transport the heart to the implant centre to ensure a tolerable ischaemic time. There is an inverse relationship between ischaemic time and post-transplant survival.4
Heart transplantation: operative steps
Through a mid-line sternotomy, the diseased heart is exposed. Following full systemic heparinization, cannulation for cardiopulmonary bypass is accomplished with a straight aortic cannula, high in the ascending aorta. Venous cannulation is via a right angle or straight cannula directly into the superior vena cava or through the posterior right atrium into the superior vena cava. Inferior vena cava cannulation is accomplished through the posterior right atrium. Certain situations, such as recipient instability or difficult medias- tinal dissection may need femoral cannulation for cardiopulmonary bypass.
When the donor heart is close to the operating room the ascending aorta is cross-clamped and the diseased heart is excised. An incision is made in the right atrial appendage. This is extended inferiorly, anterior to the inferior vena cava cannula and towards the aorta root. The left atrium is entered through the superior limb of the fossa ovalis. The aorta and pulmonary arteries are transected just above their ventriculo-arterial valves. The interatrial septum is divided down to the coronary sinus, which demarcates the atrio- ventricular groove. Resection of the heart results in cuffs of left atrium, SVC, IVC, aorta, and pulmonary artery.
Prior to implantation, the donor heart is inspected for a patent foramen ovale, which is closed by direct suture, and any other anomalies. Further myocardial protection in the form of 1000 ml of cold blood cardioplegia may be given into the donor heart via its clamped aortic root.
The implant procedure begins with anastomosis of donor and recipient left atria5 (Figure 1). Using a 4/0 polypropylene monofilament suture, the anastomosis starts at the level of the left atrial appendage. Care is taken to align the interatrial septum. A transmitral vent is placed prior to completion of the left atrial anastomosis to vent the left ventricle and stop it from distending. Cardiac cooling may be augmented by running cold Ringer’s solution through this vent during implantation. The next anastomosis is to the donor and recipient aorta. The donor aorta is left long so that there this is little tension on the anastomosis, and so it can be manipulated to allow inspection of the suture lines on completion of the transplant. This is done with continuous 4/0 polypropylene monofilament suture. At this point the heart can be deaired via a needle vent placed in the ascending aorta, and reperfusion begun with warm, leukocyte filtered blood at a carefully controlled pressure. Prior to release of the cross clamp, 500 mg of methylprednisolone is given intravenously. The next anastomosis is the PA, this is done with continuous 5/0 polypropylene. The pulmonary artery should be trimmed short. If the pulmonary artery is left too long, distortion can occur with re-establishment of normal right ventricular contraction. The final anastomoses are between the respective inferior and superior vena cavae,6 with continuous 5/0 polypropylene, tied down initially to prevent purse stringing of these low pressure structures.
When the patient has been rewarmed there follows at least 30 min of reperfusion. An isoprenaline infusion is commenced, pacing wires are placed on the surface of the heart and the patient is weaned from cardiopulmonary bypass. Protamine is administered to reverse the effects of heparin and haemostasis is performed.
The patient is managed in an intensive care environment in an isolated room. The patient is rewarmed and ventilatory requirements are progressively weaned. There is careful monitoring of cardiac function and volume status. This may be done with a Swan Ganz catheter or a left atrial line placed at the time of the transplant. Transoesophageal echocardiography is also used to monitor graft function and to rule out a pericardial collection or tamponade, if suspected. Immunosuppression consists of methylpredisolone 125 mg given 8 hourly for three doses and then the institution of cyclosporine at 1-2 mg/kg if there is no or minimal renal dysfunction. If there is renal dysfunction preoperatively, then a cyclosporine sparing regimen is possible using antithymocyte globulin with later institution of the calcineurin inhibitor. Eventually, the patient is established on a stable regimen of oral corticosteroid, calcineurin inhibitor, typically ciclosporin or tacrolimus, and an antimetabolite, usually azathioprine or mycophenolate. The International Society of Heart and Lung Transplantation (ISHLT) have issued guidelines which cover all subsequent management.7
Results
The registry database is managed by the International Society of Heart and Lung Transplantation. Since 1967, in excess of 88,000 total heart transplants through to the end of March 2010.4 In this cumulative registry, 1-year survival is 81%. Thereafter the annual mortality is 4% per year. The half-life for survival is 10 years. Hazards are highest in the first year of transplant, after this the conditional half-life is 13 years. Analysis has shown that more recent cohorts have a better survival. The causes of death within the first 6 months are mainly due to graft dysfunction and infection. Late attrition is due to chronic rejection, in particular chronic allograft vasculopathy and malignancies. Improved outcome by era of transplantation has been shown and is due to a number of factors including the emergence of calcineurin inhibitors, lower chronic steroid use, earlier diagnosis of rejection and better patient selection.
Lung transplantation
Indications for lung transplantation
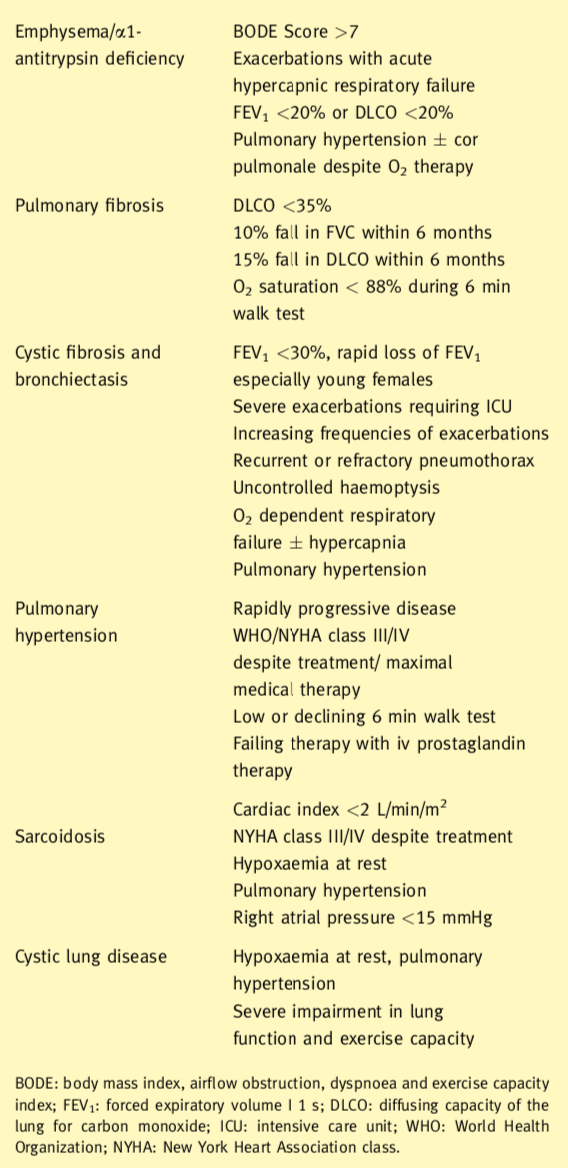
Indications for lung transplantation include in general terms obstructive, septic, restrictive and vascular pulmonary diseases. The eligibility criteria for consideration for transplantation also are shown. In the presence of septic lung disease or pulmonary hypertension, the patient should receive a bilateral lung transplant. This removes the focus of sepsis and prevents contamination of the transplanted lung and provides the largest possible vascular bed. Lung transplant candidates with obstructive lung disease or pulmonary fibrosis may receive either a single (SLT) or a bilateral lung transplant (BLT).
Preoperative preparation
Typically donor-recipient matching takes place on the basis of blood group, predicted and measured total lung capacity, excluding those recipients with preexisting antibodies to the donor’s HLA antigens. The donor lungs are assessed by bronchoscopy. In those heart beating brain stem dead patients, the function of the lungs can be assessed by measuring pulmonary venous gases (PO2) from superior and inferior veins from both sides once the chest has been opened and the donor lungs fully recruited at FiO2 of 100%.9 PO2 of 40 kPa and above, from each of the pulmonary veins sampled, indicates good function of the prospective donor lung. This assessment step is not possible in circulation-arrested donors (Donation after Circulatory Death, DCD). If the donor is deemed to be satisfactory, the patient is prepared for surgery. The timing of surgery is co-ordinated with the timing of organ retrieval. Immunosuppression is given preoperatively, typically, azathioprine and cyclosporine orally 24 h preoperatively as well as the anaesthetic premedication.
Donor retrieval for lungs
The retrieval process may often take place with heart retrieval. After sternotomy, the lungs are examined for appearance and size, palpation for masses, consolidation atelectasis and trauma. Air
trapping, lacerations and bullae are noted. Pulmonary venous gases are sampled to assess function of the lungs. This step is not possible in DCDs. Once this assessment has taken place the aorta, SVC, IVC and trachea are encircled. Heparin is administered. The pulmonary artery is cannulated. Pulmonary perfusate (PerfadexTM) is prepared and the aorta is cannulated for cardioplegia. The lungs are inflated to avoid atelectasis. The SVC is clamped and the IVC divided. The heart is vented through the left atrial appendage. The heart is excised as previously described. The posterior pericardium is incised. The left lung is dissected by division of the left inferior pulmonary ligament, going upwards close to the oesophagus. The descending aorta is transacted then all the neck vessels are divided away from the trachea. The right lung is dissected starting from the inferior pulmonary ligament upwards to the azygos vein. Then all the right neck vessels are divided from the trachea. The lungs are inflated to avoid atelectasis and the trachea is stapled. PerfadexTM is given retrogradely into each pulmonary vein to wash out clot and debris from the pulmonary circulation. The lungs are placed in ice- cold saline and transported on ice.
Lung transplantation: operative steps
The least functional lung, determined by preoperative ventilation on perfusion scans, is resected and transplanted first. Replacement of the least functional lung may reduce the need for CPB. Patients for SLT are intubated with a double lumen tube directed to the contralateral side. Patients for BLT are intubated with a single lumen tube unless an off pump procedure is planned. There are a number of surgical approaches to the chest for lung transplantation. A BLT may be done via median sternotomy, anterior thoracotomies (with or without the sternum divided) or separate bilateral posterolateral thoracotomies. A SLT may be done via posterolateral or an anterior thoracotomy. We will describe the usual situation of a BLT conducted via bilateral anterior thoracotomies with sternal division (clamshell incision).
For BLT, the chest is entered the fourth interspace. The sternum is divided horizontally, with a saw. Care is taken to avoid injury to the phrenic and vagus nerves, as well as the oesophagus, and thoracic duct. The pulmonary arteries and veins are dissected free preserving the lengths of the main trunks. The right pulmonary arteries are ligated and transected beyond the first branch to the upper lobe. The vein branches are ligated and divided to save as much length as possible for the recipient atrial cuff.
The bronchus is transacted, the lung is removed from the chest and the field is prepared for implantation of the graft. The pulmo nary artery stump is mobilized centrally. The pulmonary vein stumps are retracted anteriorly and laterally to permitcircumferential opening of the pericardium. Meticulous haemostasis is performed in the posterior mediastinum as this is difficult to reach following implantation. The donor lung is placed into the chest and kept cold with iced saline and slush. The anastomosis is conducted from posterior to anterior in this following order: bronchus, left atrium and pulmonary artery. The donor bronchus is shortened to minimize the risk of ischaemia distally. Ischaemia is also avoided by minimizing unnecessary circumferential dissection of the proximal recipient bronchus. The donor bronchus is divided one cartilaginous ring proximal to the upper lobe bronchus on the right and one ring proximal to the division of the main bronchus on the left. The donor pulmonary artery is trimmed to an appropriate length and the anastomosis is created with running 5/0 polypropylene suture. This is done with precise, small suture bites to avoid any stricture. The atrial anastomosis is completed with continuous 5/0 polypropylene. A Satinsky clamp is placed on the atrium. The ligatures are cut from the recipient vein stumps and the bridge of atrium between vein stumps is divided to create the atrial cuff. The left atrial anastomosis is often the most difficult technically, as the heart is in the way. The lung is partially inflated and the pulmonary artery clamp is loosened momentarily to de-air the lung. The atrial sutures are secured and the clamps are removed. 500 mg of prednisolone is given iv at this point. The suture lines are checked for haemostasis as ventilation and perfusion is restored. An identical procedure is then done on the opposite side.
The use of cardiopulmonary bypass has decreased since the early days of lung transplantation. In the UK it is still used widely. It may avoid haemodynamic compromise as the mediastinum is manipulated and may facilitate clampless open vascular anastomoses. Increasingly in the UK, lung transplantation is conducted without bypass to avoid consequences of coagulopathy, neurological dysfunction, and renal impairment. Specific indications for the use of cardiopulmonary bypass include, haemodynamic instability, inadequately oxygenation or ventilation with one lung, increase in pulmonary arterial pressures with unilateral pulmonary artery clamping, and deterioration of right ventricular function. Full or partial bypass may be used during pneumonectomy of the second lung with implantation of the second graft to avoid perfusion of the implanted lung with the full cardiac output. Patients most likely to require CPB during transplantation are those with cystic fibrosis, primary pulmonary hypertension, and pulmonary fibrosis. For bilateral lung transplant on CPB our preference is to conduct as much dissection as possible before administration of heparin and cannulation. On full CPB both lungs are excised. After the first lung is implanted, it is left in place with the vascular clamps intact and the atrial suture line untied. The lung is packed in iced saline and slush while the second lung is implanted. We use a period of controlled reperfusion to minimize reperfusion injury for 20 min. The PA pressures are monitored and blood is allowed to flow through the lungs with a pulsatile PA arterial trace keeping a maximal PApressure of 25 mmHg systolic while ventilating with room air. After this period there is restoration of perfusion and ventilation with supplemental oxygen.10 Once stability is achieved, CPB is progressively weaned and the patient separated.
Early postoperative management
These patients are managed in isolation in the intensive care unit. Ventilatory support is carefully managed to limit inspiratory pressures (less than 35 cm H2O) and in the case of SLT keeping peak end expiratory pressures low, to avoid overdistension of the native lung. There is early physiotherapy, and tracheostomy if prolonged extubation is expected, as this improves mobility and expectoration. Fluid, cardiovascular and renal management aims to maintain the patient in a negative balance. There is a strategy to replace volume with blood and colloids and many patients may require vasopressors. Immunosuppression is commenced as soon as the patient arrives in the intensive care unit (methylprednisolone 125 mg 8 hourly and ciclosporine iv).I n the first week, the major complications are reperfusion injury and infection. In the second week, the predominant complications are rejection and infection.
Primary graft dysfunction
Primary graft dysfunction (PGD) accounts for 50% of the 30-day mortality associated with lung transplantation. It is a syndrome associated with impaired oxygenation and pulmonary infiltrates. Its mechanism is not completely understood but may be due to the level of injury sustained by the donor lung, reperfusion injury, and devices (Figure 3a and b). Their mid-term and long outcomes are less favourable than transplantation, and device failure, thrombosis and infection are still very much a problem.14 To overcome the critical organ shortage especially for lungs, many transplant pro- grammes have relaxed their ideal donor criteria and by using extended-criteria donors, DCDs, and ex-vivo perfused lungs.
Treatment of these patients consist of sedation, elevated positive end expiratory inflation pressures and lower peak inspiratory pressures. Excessive fluid administration is avoided. Inhaled nitric oxide may also be used as well as prostaglandin E1. Severe primary graft dysfunction may also be treated with extra corporeal membrane oxygenation.
Results
Since 1988 the ISHLT has collected data from the majority of lung transplantation centres world wide, voluntarily. In the year ending March 2010, 2769 single and double lung transplants were reported. The mortality rate is highest in the first year. For all lung transplants the half-life reported was 5.3 years, excluding the first year of attrition (conditional half-life) 7.5 years. The 1- and 5-year survival for BLT is 6.6 and 9.9 years respectively and for SLT 4.6 and 6.4 years. These are unadjusted figures. Rates of survival vary for patient age, type of transplant procedure and underlying diagnosis. COPD and IPF are associated with reduced survival probably because of the advanced age of this recipient population and the increased likelihood of receiving a SLT. Best survival is seen with younger patients receiving a BLT, whereas the worst outcomes are seen with elderly patients with IPF and SLT.
As a group, lung transplantation provides survival benefit for patients with end stage pulmonary disease. This is best seen in patients with CF or IPF, and improved survival can be shown within a few months compared to ongoing medical management for individuals eligible for transplantation. It can be concluded, therefore, that transplantation is most beneficial in the preterminal phase of these illnesses. In general patients are listed when their life expectancy falls below 2 years
www.uktransplant.org.uk/ukt/statistics/calendar_year_statistics. 31-3-0010.
Mehra MR, Kobashigawa J, Starling R, et al. Listing criteria for heart transplantation: International Society for Heart and Lung Trans- plantation guidelines for the care of cardiac transplant candidates 2006. J Heart Lung Transplant 2006 September; 25: 1024-42.
Aaronson KD, Schwartz JS, Chen TM, Wong KL, Goin JE, Mancini DM. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation 1997 June 17; 95: 2660-7.
Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty- seventh official adult lung and heart-lung transplant report e 2010. J Heart Lung Transplant 2010 October; 29: 1104-18.
Aziz T, Burgess M, Khafagy R, et al. Bicaval and standard techniques in orthotopic heart transplantation: medium-term experience in cardiac performance and survival. J Thorac Cardiovasc Surg 1999 July; 118: 115-22.
Costanzo MR, Costanzo MR, Dipchand A, et al. The International Society of Heart and Lung Transplantation guidelines for the care of heart transplant recipients. J Heart Lung Transplant 2010 August; 29: 914-56.
Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update e a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2006 July; 25: 745-55.
Botha P, Rostron AJ, Fisher AJ, Dark JH. Current strategies in donor selection and management. Semin Thorac Cardiovasc Surg 2008; 20: 143-51.Clark SC, Sudarshan CD, Dark JH. Controlled perfusion of the transplanted lung. Ann Thorac Surg 2001 May; 71: 1755e6.
Christie JD, Van RD, de PM, et al. Report of the ISHLT Working Group on primary lung graft dysfunction parts I-6. J Heart Lung Transplant 2005 October; 24: 1451-500.
Geertsma A, Ten Vergert EM, Bonsel GJ, de Boer WJ, van der Bij W. Does lung transplantation prolong life? A comparison of survival with and without transplantation. J Heart Lung Transplant 1998 May; 17: 511-6.
Hosenpud JD, Bennett LE, Keck BM, Edwards EB, Novick RJ. Effect of diagnosis on survival benefit of lung transplantation for end-stage lung disease. Lancet 1998 January 3; 351: 24-7.
Van RD, Neyrinck A, Verleden GM, et al. Lung donor selection and management. Proc Am Thorac Soc 2009 January 15; 6: 28-38.
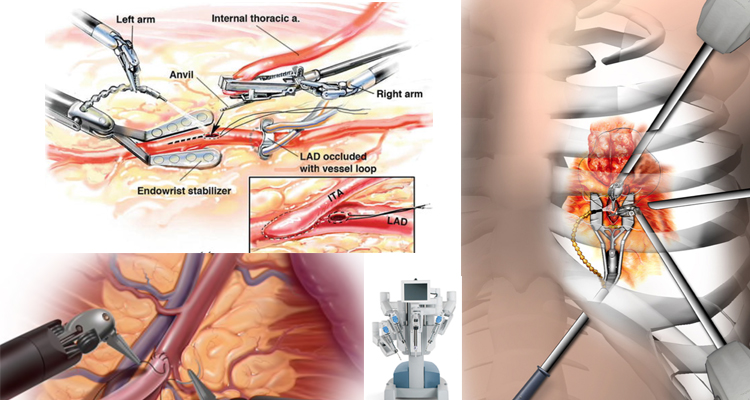
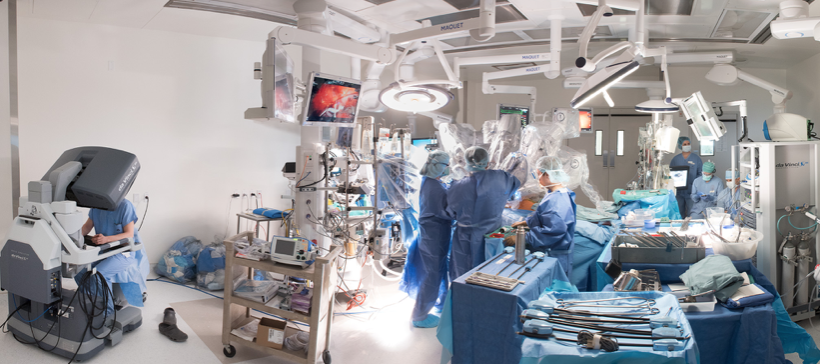
Robotic cardiac surgery is heart surgery done through very small cuts in the chest. With the use of tiny instruments and robot-controlled tools, Dr Babar Bashir CHAUDHRI is able to do heart surgery in a much less invasive way than conventional open-heart surgery.
Robotic surgeries have been used for a number of different heart-related procedures, including valve surgery, especially mitral surgery, coronary artery bypass, cardiac tissue ablation, heart defect repair, and tumour removal.
The advantages include the need for smaller incisions, rapid recovery and the need for fewer blood transfusions. From the surgeon’s perspective the advantages are excellent exposure and the robotic instruments can mimic the human hand’s range of movements within small spaces.
Robotic surgery is not applicable for every patient, emergency surgery should still be done via open approaches, because they are much quicker and time is important for rapid and successful recovery under these circumstances and some structures are difficult to operate with this approach, especially the aorta and the aortic valve.
Dr Babar Bashir Chaudhri is collaborating with experts World-wide (New York, Glasgow, Tel Aviv, Vienna) to bring the latest robotic operative techniques to treat his patients.